In In-depth
Follow this topic
Bookmark
Record learning outcomes
Nearly one in two people in the UK are affected by cancer in their lifetime. In April 2025, NHS England rolled out a new ‘super-jab’ for 15 different types of cancers, being the first in Europe to offer this new injection. The new under-the-skin (subcutaneous) nivolumab injection, which has been approved by the Medicines and Healthcare products Regulatory Agency (MHRA), means faster and more convenient treatment for NHS cancer patients and reduced pressure on the overstretched NHS. The roll-out is said to save over a year’s worth of treatment time for patients and NHS teams annually.
Background
The MHRA approval is based on evidence from a randomised, open-label Phase 3 clinical trial (called CheckMate-67T), involving patients with a specific form of advanced or metastatic clear cell renal carcinoma (a type of kidney cancer). In the trial, patients received either the new subcutaneous injection of nivolumab or the established intravenous (IV) nivolumab infusion.
Results showed that the injection produced comparable levels of the drug in the body and a similar safety and tumour response profile to the IV formulation. Patients in the clinical trial said that they preferred the new injection to the established IV version.
“A recent study called CheckMate-67T showed that the new under-the-skin injection of nivolumab works just as well as the traditional IV drip,” says Dani Edmunds, research information manager at Cancer Research UK.
“Anything we can do to make treatment more tolerable and convenient for people with cancer is a step in the right direction. The jab could reduce the time patients spend in hospital and give them more time to spend at home with loved ones – and that can make a real difference to quality of life.”
What is nivolumab?
Nivolumab (Opdivo) is an immunotherapy treatment that uses the patient’s own immune system to destroy cancer cells. It’s a type of monoclonal antibody and is also called a checkpoint inhibitor drug. The drug works by binding to a checkpoint protein called PD-1 (programmed cell death-1 protein). PD-1 is found on T-cells, which are an essential component of the immune system.
Checkpoint proteins either turn on or turn off the immune system to keep it working properly. During an immune response, PD-1 turns off the immune system before it overreacts and attacks the body’s healthy cells and tissues. Some cancer cells make high levels of PD-1, stopping the immune system from attacking them. By blocking PD-1, nivolumab turns the immune system back on so that T-cells can detect and destroy the cancer cells.
Nivolumab IV infusions are already being used to treat a range of cancer types – including melanoma, lung, kidney and head and neck cancers, as well as some types of bowel and stomach cancers. “Cancer Research UK helped pave the way for immunotherapies such as nivolumab through decades of research,” says Dr Edmunds. “We helped test nivolumab in a major clinical trial for people with mesothelioma, a rare and hard-to-treat cancer.
It was the first treatment shown to help people with this disease live longer after chemotherapy stopped working. Researchers are still investigating how we can use nivolumab and other similar drugs to treat more people with more types of cancer.”
“It could save NHS staff around 1,000 hours of treatment time every month... That's time that could be spent helping more patients, sooner”
Patient benefits
The IV infusion of nivolumab is already one of the most widely used first-line cancer treatments. However, this is a time-consuming process and requires a permanent access (infusion) point under the skin, which can cause complications.
Each infusion through an IV drip takes 30-60 minutes, depending on the cancer type. This needs to be given in a specialist treatment centre, which can mean that patients are travelling a long way for each appointment, usually every two to four weeks.
The new nivolumab subcutaneous injection is given directly into fatty tissue under the skin. It takes just three to five minutes to administer, has a shorter preparation time and should reduce pain and discomfort. This means that although patients will still need to visit the hospital every two to four weeks for treatment, they won’t need to spend so much time there, even taking into account the observation time afterwards.
The treatment also won’t need to be given at specialist treatment centres. Cancer experts hope that the subcutaneous injections will enable patients to access nivolumab treatment closer to home, and clinical trials are researching whether this could eventually be used as an ‘at-home’ treatment in the future.
Hospital benefits
Around 1,200 patients in England per month are expected to benefit from the new nivolumab injections. Two in five patients who currently receive nivolumab IV infusions should be eligible for the new jab, and most eligible new patients are expected to begin on subcutaneous nivolumab rather than the infusions.
The nivolumab injections may be given on their own or alongside other cancer treatments, including chemotherapy. Their use comes at no extra cost to the NHS thanks to an agreement negotiated by NHS England with the manufacturer Bristol Myers Squibb.
A major benefit for the NHS is that the new injection will provide greater flexibility and mean that several patients can be treated with the injections in the same time frame as one patient having the infusions. “Because the jab is quicker to give, it could save NHS staff around 1,000 hours of treatment time every month, according to some estimates,” says Caroline Geraghty, senior specialist information nurse at Cancer Research UK.
“That’s time that could be spent helping more patients, sooner. Innovations like this have the potential to ease pressure on services while improving care.”
Benefits in action
The Mount Vernon Cancer Centre in Northwood, Middlesex, has become the first cancer centre in the UK to treat a patient with the new nivolumab injections. “Changing how this immunotherapy drug is given will make a big difference for patients,” says Dr Heather Shaw, consultant medical oncologist (skin cancers) and the Centre’s deputy clinical director.
“They won’t need cannulas and will spend less time in the hospital, making their experience much better.”
The Mount Vernon Cancer Centre team prioritised prescribing guidelines and worked with the drug manufacturer to ensure they were ready to treat patients as soon as the injection was available in the UK.
“By administering this immunotherapy treatment through a brief injection, patient hospital visits will be shortened, enabling us to increase the number of patients treated within our day units,” says Dr Amy Guppy, consultant medical oncologist and chemotherapy lead at Mount Vernon Cancer Centre.
Safety profile
Nivolumab isn’t suitable for everyone and, like other cancer drugs, can cause side effects, some of which can be severe. Checkpoint inhibitor drugs boost the immune system by increasing the activity of all immune cells, not only those targeting cancer.
These immune cells can then attack normal (non-cancer) cells, leading to inflammation in different parts of the body, during or after treatment. Delayed side effects can start weeks, months or even more than a year after the treatment has finished.
Some general side effects of checkpoint inhibitors include allergic reactions, dry skin and rashes, headaches, diarrhoea, sickness or abdominal pain, fatigue, breathless and a dry cough.
These drugs can also affect blood sugar levels and the liver, kidneys, heart and hormone-making glands such as the thyroid. Patients therefore need to have regular blood tests.
“The CheckMate-67T trial found that people receiving the injection didn’t experience any more side effects than those on the IV version, even months after their last dose,” says Caroline. “That means patients shouldn’t need extra monitoring, and safety shouldn’t be compromised by switching to the jab.”
If anyone is being treated with nivolumab injections or infusions and notices any discomfort or changes in how they feel, they should consult their doctor, nurse or pharmacist and report it directly to the MHRA Yellow Card scheme. “We’re assured that the appropriate regulatory standards of safety, quality, and efficacy for the approval of this new formulation have been met,” says Julian Beach, MHRA interim executive director of healthcare quality and access. “As with all products, we will keep its safety under close review.”
Red flag symptoms
Cancer symptoms usually depend on the type of cancer (and subtype) and can vary from person to person. There are, however, certain red flags that pharmacy teams should look for when discussing symptoms with customers at the pharmacy counter. The following symptoms all require urgent referral to a GP, although these may not turn out to be a sign of cancer. Customers should also see a GP if they have any other symptoms or changes that are unusual for them or if they’re worried that existing symptoms could be due to cancer.
According to the NHS website, general red flag symptoms for cancer include:
- A new lump or swelling anywhere on the body
- Sweating a lot (particularly at night) or a high temperature that lasts a long time or keeps coming back
- Feeling more tired than usual
- Unusual bruising or bleeding (such as bleeding from the anus, blood in the urine or vomiting blood)
- Pain anywhere in the body that’s not usual for them
- Losing weight without trying or feeling less hungry than usual
- Needing to urinate more often or more urgently, or pain when they urinate.
Some cancer symptoms affect specific parts of the body.
- Skin cancer symptoms may include new moles or changes to a mole, or a rash or sore that doesn’t improve
- Digestive cancer symptoms may include severe, regular or worsening heartburn or indigestion, a bloated tummy that happens regularly or isn’t going away, and changes to bowel movements that don’t get better or get worse
- Mouth and throat cancer symptoms may include problems swallowing, a constant hoarse or croaky voice, a sore or ulcer in the mouth that doesn’t get better, and white or red patches in the mouth
- Lung cancer symptoms may include a persistent or worsening cough or shortness of breath and coughing up blood
- Vagina and penis symptoms can include vaginal discharge that is unusual for them, unusual vaginal bleeding, difficulty getting an erection and blood in the semen.
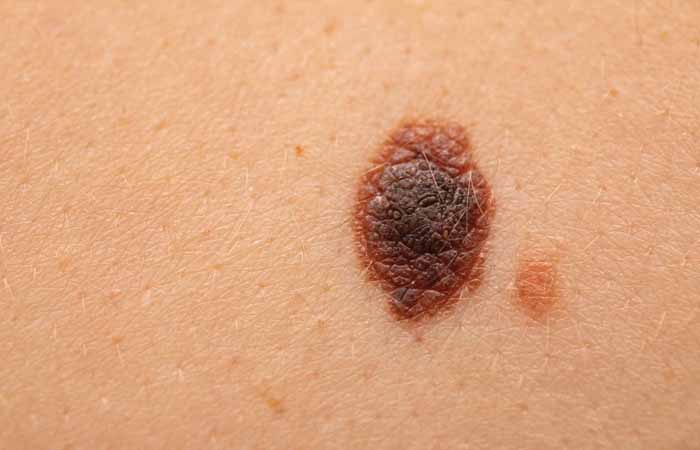
Skin cancer symptoms may include new moles or changes to a mole.