Covid-19: vaccination nation
In In-depth
Follow this topic
Bookmark
Record learning outcomes
As the rollout of the UK’s Covid-19 vaccination programme steps up, find out what pharmacy teams and their customers need to know next
The UK’s network of Covid-19 vaccination sites has rapidly expanded since the nationwide vaccination program launched in January 2021. It now encompasses over 1,500 locations made up of hospital hubs, vaccination centres and some 1,200 local sites including community pharmacies.
The Government’s aim is for everyone to live within 10 miles of a vaccination centre, in order to meet its target capacity of vaccinating at least two million people per week, and the plan seems to be running smoothly so far. At the time of writing, over 20 million people – around one in three adults across the UK – have received their first dose of the vaccination, with almost a million having had their second dose, and this is increasing every day.
As the return of spring is welcomed, the UK’s vaccine rollout is set to step up to the next phase. Everyone in the top four priority groups has already been offered at least one dose of the jab, which includes most frontline health and social care workers, elderly care home residents, clinically extremely vulnerable people and the over-70s. This means the next tranche of people – the over-60s, anyone over 16 with a health condition which increases their risk, adult carers of disabled people, and younger adults in care homes – are now being invited for vaccination.
These will be followed by all those aged 55 and over, then the 50 and over age group, and finally the rest of the population. Final plans for this are still to be determined, but the Government’s ambition is for the whole adult population, in England and Wales at least, to have been offered their first dose of the vaccination by the end of July.
Vaccine development
It has been over a year since scientists in China decoded and shared the genetic sequence of the SARS-CoV-2 virus that causes Covid-19 with fellow researchers across the globe. Since then, labs around the world have been working on creating effective vaccines, each working in slightly different ways:
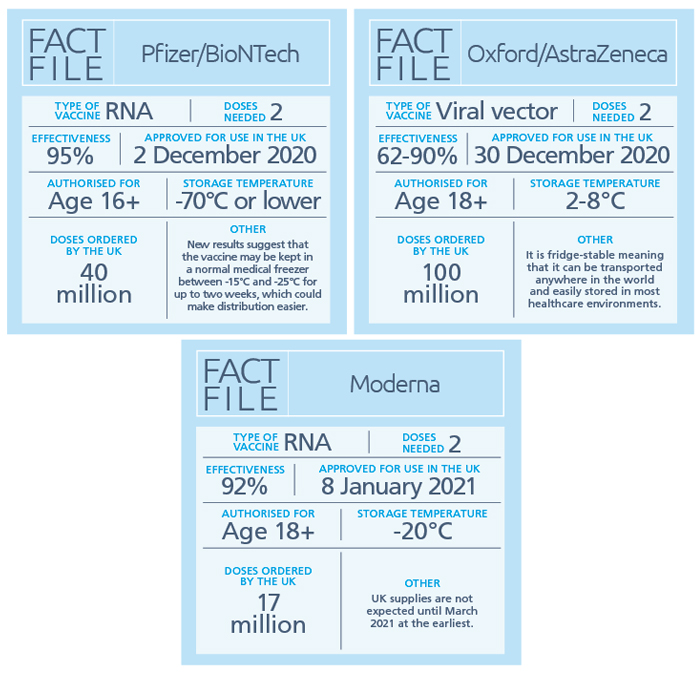
- RNA or DNA (nucleic acid) vaccines use genetic material from the ‘viral spike protein’ of the SARS-CoV-2 virus. This vaccine encourages human cells to make the antigen that will trigger an immune response. A downside is that these vaccines – such as the PfizerBioNTech vaccine – have to be kept at ultra-cold temperatures of -70°C or lower
- Viral vector vaccines – such as the Oxford/AstraZeneca vaccine – also work by giving cells genetic instructions to produce antigens, using a harmless virus such as the one that causes the common cold, to deliver these instructions into the cell
- Live attenuated vaccines use a weakened form of the virus that can still replicate without causing illness, and inactivated vaccines use viruses that cannot infect us but can still trigger an immune response. There is a risk of live attenuated vaccines causing disease in people with compromised immune systems, but inactivated virus vaccines can be given to these patient groups
- Protein subunit vaccines use pieces of the virus protein to trigger an immune response. However, because the immune response may be weaker, these vaccines often include adjuvants – substances that enhance the body’s immune response.
Vaccine contenders
The Medicines and Healthcare products Regulatory Agency (MHRA) has so far approved the Pfizer/BioNTech, Oxford/AstraZeneca and Moderna vaccines and has ordered almost 160 million of these so far. However, at the time of writing, other vaccines are still in development or being considered for regulatory approval, and are also on the Government’s shopping list.
Johnson & Johnson has reported that its one-dose viral vector vaccine is 66 per cent effective at preventing moderate to severe illness 28 days after vaccination. The UK has agreed a deal for 30 million doses.
The two-dose Novavax protein subunit vaccine developed in the USA has been shown in clinical trials to be 95.6 per cent effective against the original variant of SARS-CoV-2, but also to provide protection against the newer UK variant (85.6 per cent) and the South African variant (60 per cent). The UK has secured 60 million doses.

Dispelling myths and misconceptions
Despite all the positive news about the vaccines, and clear and open information about their development and approval, the internet has become a hotbed of misinformation, from suggestions that the vaccines may impact fertility, to warnings that it will induce worse side effects than the virus itself.
These unsubstantiated claims have led many people to feel hesitant about taking the vaccine. Indeed, a recent survey commissioned by Eskenzi PR and conducted by Censuswide, found as much as 40 per cent of the UK population has been put off taking the vaccine due to what they have read in the media.
Dr Mahendra Patel, RPS board member and pharmacy research champion NIHR (Yorks & Humber), has been working hard to demystify some of the misconceptions and misinformation – especially among black, Asian and ethnic minority (BAME) communities – about Covid-19 and the vaccines. He says there are lots of questions that pharmacy teams are well placed to answer in order to put customers’ minds at rest, but adds: “These myths linger and can be damaging so your messaging has to be evidence-based and factual, not anecdotal or your opinions.”
Here are some common questions that pharmacy teams are being asked, along with some appropriate answers:
Q: How safe is the Covid-19 vaccine?
A: In order to be approved for use, every Covid-19 vaccine must go through all the clinical trials and safety checks of every other licensed medicine. The vaccines currently being used in the UK meet strict standards of safety, quality and effectiveness set out by the MHRA.
Q: How come the vaccines were made so quickly?
A: Mahendra says: “The science and the knowledge behind the vaccines are basic stuff, but extra funding has accelerated the research, and researchers were put on the job from other projects, which has cut through the usual timescales.”
Q: What if I’m allergic to the vaccine?
A: The vaccines do not contain any animal products or egg, and it is very rare for anyone to have a serious reaction. If this does happen, it usually happens within minutes, and vaccinators are trained to deal with allergic reactions immediately.
Q: Can I have the vaccine if I am pregnant or breastfeeding?
A: The NHS says although there is no evidence that the vaccine is unsafe if you’re pregnant or breastfeeding, more evidence is needed and so pregnant or breastfeeding women should wait to have the vaccine. However, the Government’s Joint Committee on Vaccination and Immunisation (JCVI) has advised that pregnant women should consider having the Covid-19 vaccine if they are clinically extremely vulnerable or frontline health or social care workers.
Q: What are the possible side effects?
A: So far, thousands of people have been given the Covid-19 vaccines in clinical trials and in real life with no serious side effects or complications reported. The vast majority of side effects reported are mild and in line with most types of vaccine, including the seasonal flu vaccine. These include sore arms and mild ‘flu-like’ symptoms, which reflect a normal immune response to vaccines.
Q: How effective is the Covid-19 vaccine?
A: The Pfizer/BioNTech vaccine is 95 per cent effective against Covid-19, while the figure for the Oxford vaccine is between 62 and 90 per cent. This is still a better result than the best flu jab, which is about 50 per cent effective. However, this means that even once vaccinated there is still a small chance someone might still get Covid-19 so it is important to continue to follow social distancing guidance, and wear a face covering if able, in places where it’s hard to stay away from other people.
Q: Is getting the Covid-19 vaccine really worth it?
A: The latest analysis of the approved Covid-19 vaccines by the MHRA shows that their safety remains as high as expected from the clinical trial data that supported the approvals. According to the MHRA, “the benefits continue to far outweigh any known side effects”, and Mahendra adds that “we have yet to see somebody being hospitalised or dying out of all the thousands of patients the vaccines have been trialled on”.
Pharmacy teams getting on board
As more community pharmacies become involved in the vaccination program, the role for pharmacy teams is increasing, and some are even training as vaccinators themselves – see TM's new 'Pandemic Protection' series.
Avicenna opened a vaccination clinic at its Swindon pharmacy at the start of February, turning around a refit in a matter of days to include vaccination pods, a reception and waiting areas. The clinic aims to vaccinate 800 patients a week, and Nazlin Meghji, regional lead of Avicenna Pharmacies, says the pharmacy team is crucial for the success of the operation.
“As well as getting on with their day-to-day business, the [Swindon] pharmacy’s core team are particularly busy answering phone calls from customers concerned about what to expect from their vaccination, and we have trained our counter staff and our dispenser to listen sympathetically and answer any queries,” she explains.
Nazlin says one of the biggest issues in Swindon and the wider South West region is hesitancy among the BAME community to take up their vaccination. “Luckily we have a counter assistant who has relationships with these communities, and we asked her to tell her friends and family that they can ask us any questions and we can set their minds at rest,” says Nazlin. “In the first few weeks of vaccinating in Swindon, we didn’t see any BAME community members coming in, but that is changing since she has been spreading the word.”
Everyone in the top four priority groups has already been offered at least one dose of the jab
Supporting customers
Kellaway Pharmacy in Westbury Park, Bristol, also opened its vaccination clinic at the start of February, running 12 hours a day, seven days a week.

Pharmacy technician Shasha Eva Peng completed her vaccination training and within the first couple of days of the clinic opening had already vaccinated 200 patients. “This is my first time vaccinating,” says Shasha. “But luckily the first person I vaccinated was my pharmacist, Yolanda Kong, which put me at ease.”
Shasha says she has seen that queries from customers about the vaccination programme have changed since it has progressed. “At the start of the programme, people’s questions were about how safe the vaccines were, how well they had been tested and whether they would make them sick. Now, the most common query we get is whether someone is eligible for the vaccination yet. If they are not, then we explain why and talk them through how to book their place when they are,” she explains.
Another challenge has been the booking system, which Shasha says has proved tricky for some of the more elderly patients who haven’t realised that they need to scroll down the page and click a button to confirm their chose slot. “We have had quite a few people turn up on the day they expect to be vaccinated only to find they are not booked in, and we’ve worked out this is why. This is stressful for them, so one thing I’ve done to help them is taken a screenshot of what a completed booking page looks like so they know how to finish the process when they have to do it again.”
Mahendra says it’s wide-ranging advice like this that can make all the difference to patients’ experiences. “As an HCP you are an ambassador and a public health champion,” he says. “The more information you have to hand, the better equipped you are to share that knowledge.”
